EPDM
Rubbers > Group M
Etileno Propileno Dieno Monómero Caucho (EPDM) | ||||||||
EPDM is a terpolymer based on three monomers: ethylene, propylene and an unconjugated diene (ethylidene norbornene ENB) The EPDM grades have a residual unsaturation in the side chains and, therefore, can be cured with sulfur and accelerators. Its resistance to heat is clearly better than natural rubber, SBR and butadiene rubber. EPM represents a copolymer of ethylene and propylene monomers. EPM is completely saturated and therefore requires vulcanization by radiation or products that release free radicals, such as organic peroxides. The molecules of both EPM and EPDM have a fully saturated hydrocarbon bone, which is excellent heat resistance and oxidation is achieved. | ||||||||
Symbols | Estructura | |||||||
|  | |||||||
| Characteristics of rubber | ||||||||
|  | |||||||
| Physical-Mechanical Properties | ||||||||
The properties are directly related to the polymerization conditions. By varying the reaction parameters, a wide range of properties can be obtained. The production of a wide range of qualities of synthetic rubber of this type is possible by a deep understanding of the relationship between the process variables and the properties of the resulting product. Influence of the ethylene / propylene ratio If the ethylene and propylene contents are about 50/50%, both monomers within the polymer molecule are evenly distributed, which means that the rubber is amorphous. If the ethylene content is greater than 64% by weight, increasing number and length sequences are formed, these sequences form crystallites. In vulcanization, the crystallinity of the polymer improves the tensile strength and increases the hardness, it also improves the set compression at low temperatures, good extrudability and improves the filling capacity of plasticizer and filler. | ||||||||
| How synthetic rubber is obtained | ||||||||
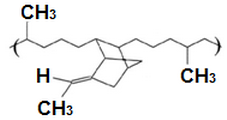
EPDM terpolymers To produce a sulfur-curable rubber, a non-conjugated diene is introduced as the third monomer during the polymerization. The third appropriate monomers contain a double bond that participates in the polymerization and another that does not. These results are in contrast to the unsaturation that serves as crosslink sites without affecting the saturated backbone. EPDM terpolymers use ethylidene norbornene (ENB) as the third monomer. EPM copolymers EPM copolymers are produced by copolymerization of ethylene and propylene using Ziegler Natta catalysts which are formed in situ by reaction of vanadium salts and alkyl aluminum halides. At high levels of propylene (above 50 weight percent), only short ethylene sequences exist in the polymer chain and crystallinity is absent. At low levels of propylene (for example, below 35 weight percent), a small amount of crystallinity is present that provides the EPM with green strength EPM vulcanization The copolymerization results in a saturated skeleton. Sulfur vulcanization can not be used to cross-link EPM since there is no unsaturation. Therefore, for example, peroxide curing should be used. EPM peroxide curing is more efficient in compositions with high ethylene content, two to be a competitive chain cleavage that occurs in the tertiary carbon atoms of the propylene units. | ENB VNB 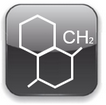 | |||||||
| Vulcanization | ||||||||
The range of EP grades consists of EPM copolymers and EPDM terpolymers. The copolymers can only be crosslinked with peroxides or radiation. The terpolymers can be crosslinked with both peroxides and sulfur. The rate of healing and cross-linking. Terpolymers with high levels of ENB are particularly suitable for the production of profiles by continuous pressure. Cured with peroxide Both EPM and EPDM can be vulcanized with organic peroxides. The choice of peroxide and coagent is important to achieve the optimum curing rate for processing conditions. The amount of peroxide has little influence on the cure rate but will influence the final crosslink density. Peroxides give the most stable cross-links to heat. A fairly high proportion of ethylene / propylene in the polymer contributes to the crosslinking efficiency. When the free radicals formed by the peroxide attack a propylene unit, there is a much greater tendency to break the chain than to cross-link. Therefore, more cross-links are formed per mole of peroxide in an EPDM with high ethylene content than in an EPDM with low ethylene content. Sulfur cure For curing with sulfur, the amount of thermonomer in the EPDM polymer (unsaturation in the side chain) determines the rate of crosslinking. However, even at high levels of thermonomers, EPDM has a lower reactivity compared to natural rubber or butadiene rubber, and therefore, accelerators must be used to achieve cure times that are acceptable in practice. Care should be taken when designing the accelerator package, since many have limited solubility in the EPDM, which may result in a surface blooming. | ||||||||
| Applications | ||||||||
EP (D) M rubber is used in a wide variety of application ranges from automotive radiator hoses, window packing tapes, cover membranes and cable insulation. It is also used as an impact modification modifier in plastics, vulcanized TPV thermoplastics and additives for motor oil. The versatility in the design and performance of the polymer has resulted in the wide use in gasketing joints for cars, weather strips for glass, garden and irrigation hoses, pipes, belts, electrical insulation, roofing membranes. | ||||||||
