Moisture absorption
Polypedia > Defects in polymers
| Humidity | |
| Plastics hygroscopics | |
In particular, in non-hygroscopic polymers moisture is retained on the surface, while in hygroscopic plastics, moisture is also absorbed inside the plastic granule. Moisture, both external and internal, negatively affects the aesthetic and functional quality of the artifact; In fact, the transformation temperatures of polymeric materials, water can be converted into water vapor, resulting in the formation of: Stripes Bubbles from the surface Irregular withdrawals Structural stress Deformation Breaks Matte appearance Weak welding lines Incomplete pieces Stains Water, the melting temperature of the polymer, which can react rapidly with the molecular chains of the polymer itself and cause the chains to split and, consequently, a decrease in molecular weight. (Increases fluidity and lowers viscosity) | |
| Determination of humidity due to loss of | |
A dedicated moisture analyzer such as the thermo-scale, is frequently used in the industry for quality control, production, inspection and receipt of solid polymers. The drying process is carried out with infrared energy using a halogen bulb or a tubular metal heating element. Advantages: Speed Disadvantages: Low precision (also removes solvents and additives) |  |
| Vacuum reagent | |
The measurement volume consists mainly of the sample containers, which contains the sample and the reagent (calcium hydride). The actual weight is introduced into the instrument. The sample is placed in the sample container, which was then evacuated in a vacuum pump, approx. 30 seconds. The sample container is then heated to the selected temperature (between 60 ° C to 200 ° C). The water that evaporates reacts with the calcium reagent hydride and generates hydrogen; The gas pressure is proportional to the water content in the sample and is controlled by means of a piezoelectric transducer. CaH2 + 2 H2O → Ca (OH) 2 + 2 H 2 The team calculates the relationship between the sample pressure and the sample weight in terms of H2O content, and is shown as a percentage or in parts per million (ppm). Volatiles other than water do not react with the reagent and will condense, thus not influencing the reading. |  |
Determination of water using the Karl Fischer method | |
Unlike other techniques, this method can detect low levels of free, emulsified and dissolved water. When this method is used correctly, it is capable of measuring water levels as low as 1 ppm or 0.0001% by volume. There are two types of Karl Fischer titrators: coulometric titrators and volumetric titrators. The main difference between the two is that in the volumetric method the titrant reagent is added directly to the sample by means of a burette. Conversely, with the coulometric method, the titrant reagent is generated electrochemically in the titration cell. The coulometric method measures much lower water levels than the volumetric method. In the coulometric titration, the iodide (I2) is generated electrochemically from the iodine (I-). When the iodide (I2) makes contact with the water in the sample, the water is titrated. Once all available water has reacted, the reaction is complete. The amount of water in the sample is calculated by measuring the amount of iodide (I2) generated electrochemically from the iodine (I-) according to the following reaction: |
| Humidity | |
| Polymers | |
All polymeric materials during synthesis, transport and storage have a tendency to retain moisture, reaching an equilibrium value with the environment, which depends on the type of polymer, humidity and air temperature, size of the granule or the piece. In general, the hygroscopicity of a polymer is related to the polarity of the chemical structure of the macromolecules of the polymer itself. An important characteristic of water is given by the polarity of its molecule, with molecular dipole moment equal to 1,847 D. The water molecule forms an angle of 104.5º with the oxygen atom in the vertex and the two hydrogen atoms in both extremes. | 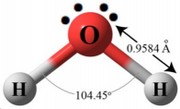 |
| Electronegativity | |
Since oxygen has a higher electronegativity, the vertex of the molecule has a negative electric charge (δ-), while the ends carry a positive electric charge (δ +). A molecule that exhibits this imbalance of electrical charges is said to be an electric dipole. In the molecular structure of many hygroscopic polymers the carbonyl group is present, which is a functional group consisting of a carbon atom and an oxygen atom bonded by a double bond. The special feature of this group is that oxygen is electronegative. | 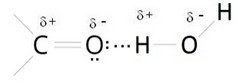 |
| Humid air | |
Humid air is a binary mixture (of dry air and water vapor). The amount of water vapor can vary from 0 (dry air) to a maximum that depends on the conditions of pressure and temperature; in the latter case, saturation is spoken (a state of equilibrium between moist air and the condensed water phase). The molecular mass of the water is 18.01534 kg / kmol, and the constant R is 461.52 J / kg. K. To define a state of a gaseous mixture, it is not enough for us to know the p and T, we also need to know what quantity of each component we have, for this, to define a thermodynamic state of humid air; In addition to knowing the p and T we need to know one of the following parameters: • Relative humidity. • The degree of humidity. • The degree of saturation. |  |
| Density of humid air | |
The density of the humid air is equal to the sum of the densities of water vapor and dry air at the respective partial pressures and the temperature of the mixture. |  |
| Relative humidity of the air | |
Relative humidity is the ratio between the density of the water vapor in the mixture or moist air and the density of the water vapor in a saturated mixture at the same temperature. | |
Degree of humidity of the air or absolute humidity of the air | |
The ratio of the mass of water vapor in a certain amount of humid air to the mass of dry air in that amount is called the humidity level. |  |
| Degree of air saturation | |
The degree of air saturation is the ratio between the moisture level of the mixture w and the maximum moisture level ws, or moisture degree of a saturated mixture at the same temperature. | |
| Dew point | |
The dew point of humid air is the temperature at which it becomes saturated humid air, if it is cooled to constant total pressure and constant humidity. |  |
| International standard for the determination of water absorption | |
The international standard for the determination of water absorption in plastics is ISO 62 standard. This describes the procedures for determining the amount of water absorbed by plastic samples when immersed in water or subjected to moist air under conditions controlled. The standard proposes three different ways to evaluate the plastic capacity to absorb water and each of these resin gives information on the behavior in different conditions: • Immersion in distilled water at 23 ° C • Immersion in distilled boiling water • Exposure to 50% relative humidity at 23 ° C Immersion tests provide information about the water content at saturation; The exposure Provides the moisture content of equilibrium moisture. |  |
| What's humidity? | |
It is the ratio between the amount of water vapor that an air mass has and the maximum it could have. P = Partial vapor pressure Contribution of water vapor pressure [P] = Pa Ps (T) = Saturation pressure Max value of the vapor pressure at a given temperature [PS] = Pa |  |
| Absorption of moisture in plastics | |
| Hygroscopic plastics | |
In particular, in non-hygroscopic polymers moisture is retained on the surface, while in hygroscopic plastics, moisture is also absorbed inside the plastic granule. Moisture, both external and internal, negatively affects the aesthetic and functional quality of the artifact; In fact, the transformation temperatures of polymeric materials, water can be converted into water vapor, resulting in the formation of:
Water, the melting temperature of the polymer, which can react rapidly with the molecular chains of the polymer itself and cause the chains to split and, consequently, a decrease in molecular weight. (Increases fluidity and lowers viscosity) |  |
