Polymerization
Process > Polymerization
| Polimerizations | ||||||||
Polymerization is a chemical process in which units of identical (homopolymer) molecules are joined (co-ter-polymer) one after another to form a large chain with those units. In the synthesis process, which is known as polymerization, higher polymer chains are obtained. There are two main types of polymers depending on their synthesis methods. If the monomers have double bonds between carbon atoms, they can be synthesized from addition reactions of the polymers. These polymers are known as addition polymers. In some of the polymerization reactions, when two monomers are joined, a small molecule such as water is extracted. Such polymers are condensation polymers There are many types of polymerization and several systems to categorize them. The main categories are:
| 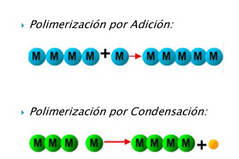 | |||||||
| Las técnicas usada en la polimerización de un monómero son: | ||||||||
| ||||||||
| Polimerización por adición | ||||||||
A polymerization is by addition if the monomer molecule becomes part of the polymer without loss of atoms. The process of addition polymers that they synthesize is known as addition polymerization. There should not be multiple monomers attached to start said reaction. This is a chain reaction; Therefore, any number of monomers can be bound in a polymer. There are three steps to a chain reaction, which are initiation, propagation and termination. For an example, we will take the synthesis of polyethylene, which is an additional polymer that is used to make products such as garbage bags, food wrappers, jars, etc. The polyethylene monomer is ethene (CH 2 = CH 2). Its unit of repetition is -CH 2-. In the initiation stage, radical peroxide is generated. This radical attacks the monomer to activate it and produce a radical of monomers. During the propagation phase, the chain grows. Activated monomer attacks of another monomer double bond and binds together. Ultimately the reaction stops when two radicals join together and form a stable bond. Chemists can control the length of the polymer chain, reaction times and other factors to obtain the required polymer. The addition polymerization does not generate by-products. |  | |||||||
| Polimerización por condensación | ||||||||
Polymerization is by condensation if the monomer molecule loses atoms when it becomes part of the polymer. A small molecule, such as water or gaseous HCL, is usually lost. Any condensation process, which results in the formation of polymers, is known as condensation polymerization. A small molecule such as water or HCl is released as a by-product during condensation polymerization. The monomer must have functional groups at the ends, which can react together to continue the polymerization. For example, if the binding ends of two molecules have a -OH group and a -COOH group, a water molecule will be released and forms an ester bond. The polyester is an example of a condensation polymer as well. In the synthesis of polypeptides, nucleic acids or polysaccharides, condensation polymerization takes place within biological systems. Condensation polymerization generates byproducts. |  | |||||||
Polimerización de crecimiento de cadena o por etapas | ||||||||
In the chain growth polymerization the monomers become part of the chain one at a time. First dimers are formed, then trimers, then tetramers, etc. The chain is increased one by one, monomer to monomer. In step growth polymerization (or steps) it is possible for one oligomer to react with others, for example a dimer with a trimer, a tetramer with a dimer, etc., so that the chain is increased by more than one monomer. In step-growth polymerization, the growing chains can react with each other to form even longer chains. This applies to chains of all sizes. In a chain growth polymerization only the monomers can react with growing chains. | ||||||||
| Cuál es la diferencia entre la suma de polimerización y polimerización por condensación? | ||||||||
Addition polymerization is the reaction between monomers with multiple bonds, where they join together to form saturated polymers. In the condensation reactions, the functional groups of two monomers react together releasing a small molecule to form a polymer. Saturated monomers are participating in the condensation reaction while for the addition polymerization, the monomer must be an unsaturated molecule. The addition polymers are non-biodegradable and difficult to recycle compared to condensation polymers. Addition polymerization is a rapid process, and it produces high molecular weight polymers, in contrast to condensation polymerization. |  | |||||||
Polimerización en masa | ||||||||
This is the simplest, homogeneous technique, where only the monomer and the initiator are present in the system. The initiation induced by thermal or radioactive effect is the most economical and the one that produces polymers of the highest degree of purity. This reaction is difficult to thermally control because it is highly exothermic (generates heat of formation). In addition, the polymer from the beginning of the reaction becomes very viscous, hindering the necessary agitation to unify the heat in the liquid, avoiding heating in certain areas. This difficulty can be avoided by initially using a pre-polymer (mixture of polymer and monomer). Produced at a lower temperature and leading to a low conversion of monomer to polymer under moderate conditions. The polymerization is completed by heating the pre-polymer at the time prior to polymerization. Mass polymerization is widely used in the manufacture of amorphous plastic lenses due to the excellent optical properties achieved in molded parts, without pressure, as in the case of polymethyl methacrylate (PMMA) Benefits: High degree of purity Requires simple equipment Disadvantage: Hard temperature control. Wide molecular weight distribution | ||||||||
| Polimerización en solución | ||||||||
This polymerization requires a solvent to dissolve the monomer and initiator and form a homogeneous system. The ideal solvent must have a low cost, low boiling point and easy polymer separation. Once the polymerization has ended, the polymer formed can be soluble or not in the solvent used. The insolubility of the polymer produces a mud that can be removed by filtration. If the polymer is soluble, a non-solvent is introduced to cause precipitation in the form of fibers or powder. The polymerization in solution has the advantage of operating with a homogeneous temperature due to the simple agitation of the system, which prevents overheating. However, the cost of the solvent and the slowness of the reaction are inconvenient. This technique is used when you want to apply the polymer solution itself and is used a lot in poly condensation Benefits: Easy temperature control. The formed polymer distribution can be used directly Disadvantage: The solvent causes reduction in weight and in the rate of reaction. Difficulties in solvent extraction | ||||||||
Polimerización en suspensión | ||||||||
The suspension polymerization is also called pearl polymerization. The polymerization is heterogeneous and the monomer and the initiator are insoluble in water which acts as a dispersing medium. The polymerization occurs within the particles of suspension of 2-10 mm of average size, and which contains the monomer and the initiator. The agitation speed determines the size of the particles. In addition, the system has active tension agents that keep the particles separate and not adhered to each other and prevent their precipitation as pearls. This effect is also improved by the addition of a water-soluble polymer of high molecular weight, by increasing the viscosity of the medium. However, these advantages are opposed to the difficulty in purifying the resulting polymer. Benefits: Easy temperature control. Obtaining the polymer in the form of pearls Disadvantage: Pollution of the polymer with stabilizing agents and water. Requires continuous agitation | ||||||||
| Polimerización en emulsión | ||||||||
Emulsion polymerization is a polymerization is a heterogeneous polymerization in liquid medium that requires a series of additives with specific functions:
The initiator is soluble in water, while the monomer is only partially soluble. This motivates the use of the emulsifier to form micelles of size between 1 mm and 1 mm formed by the monomer. Some micelles are active, because the polymerization reaction occurs inside them, while others are inactive (monomer drops). Being just a source of monomer. The progress of the reaction causes the inactive micelles to be consumed by the active ones that grow forming polymer drops and finally the solid polymer. The speed of reaction and conversion is high, and the control of the agitation and temperature is simple. The polymers obtained have large molecular weights but are of complex purification due to the large amount of additives. Benefits: Quick polymerization Obtaining polymers with high molecular weight. Easy temperature control Disadvantage: Pollution of the polymer with emulsifying agents and water | ||||||||
| Polimerización interfacial | ||||||||
Aquí, la polimerización ocurre en la interface entre dos solventes inmiscibles, en que cada uno de los monómeros esta en unas de las fases. El polímero se forma en esta interface, luego se remueve a fin de permitir la continuidad de la polimerización. Este método es limitado a un pequeño número de polimerizaciones en etapas, debido a las condiciones de reacción necesarias Beneficios: no se requiere una pureza de reactivo excesiva una relación estrictamente estequiométrica de monómeros no es necesaria altas velocidades de reacción (pocos minutos frente a horas de polimerización en masa) baja T de reacción facilidad de eliminación de productos de reacción Desventajas: es necesario recuperar el solvente orgánico necesita manejar grandes volúmenes de líquidos con monómeros muy reactivos, el rendimiento puede ser bajo debido a fenómenos de hidrólisis |  | |||||||
| Polimerización con precipitación de polímeros | ||||||||
The separation of the polymer is facilitated. The Spheripol process is important for obtaining spherical polypropylene granules. Polymerization from gaseous monomers It is polyethylene. The fluidized bed technique is used with solid catalysts on which the polymer grows. Degree of polymerization
| ||||||||
