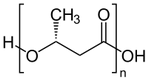
Polyhydroxyalkanoates (PHAs)
|
The polyhydroxyalkanoates (PHA) comprise a group of natural biodegradable polyesters that are synthesized by microorganisms even through bacterial fermentation or lipid sugar. Polyhydroxyalkanoates (PHAs) have very similar physicochemical properties petrochemical polymers. PHAs are thermoplastic, such as polypropylene and polyethylene, also possess antioxidant properties, optical, piezoelectric, but its properties important is that they are biodegradable and biocompatible. Its structure and therefore their properties vary widely and are a function of the producer microorgranismo, environmental conditions biosynthesis and especially the carbon source.
However, several drawbacks limit its competition with traditional synthetic plastics or their application as biomaterials ideal. These disadvantages include their high production costs, limited functionality, incompatibility with conventional techniques thermal processing and susceptibility to thermal degradation. To avoid these drawbacks, the PHA must be modified to ensure an improved performance in specific applications. In this review, summarized and discussed methods well established modification of PHAs.
| |
|
| PHA Properties |
|
|
|
- Fermentation of sugar and lipids by bacteria
- Thermoplastics or elastomers (Tm = 40-180ºC)
- Barrier to light, gases, water vapor, loss of aromas and flavors
- very brittle
- Sensitive to thermal degradation
- complicated extrusion.
- very low viscosity
- PHB: crystalline thermoplastic, very fragile
- PHBV: more flexible easier to process
- Similar mechanical properties to PP
- Properties similar to PET barrier
|
| | 
|
Range products PHA
|
|
|
More than 150 varieties of PHAs identified as homopolymers are currently known and copolymers. The reason why it is possible to form various types of PHAs is due to the wide variety of specific substrates for the synthesis of PHAs, and metabolic pathways that they activate. PHAs are biodegradable and can be produced in a sustainable and friendly way with the environment, using renewable raw materials or industrial waste. Although the advantages of PHAs are evident, the cost of production of these biopolymers is high. The two most common classes are marketed PHA: polyhydroxybutyrate homopolymer the (PHB) and polyhydroxybutyrate copolymer polyhydroxybutyrate and polyhydroxyvalerate known as valerate (PHBV).
|
| 
|
PHA Propiedades térmicas
|
|
|
The molecular mass of PHAs is about 50 ki lo-Dalton to 100 kilo-Dalton this depends on the nature of the polymer, intracellular mind can find state liquid and amorphous, that after extraction with organic solvents, this happens to a crystalline state Ríg characteristics conferring comings but turn brittle. As for the melting point is relatively high (180 ° C) but close to the thermal degradation point (200)
|
| 
|
Physical properties
|
|
|
The improved properties of PHA combined with natural materials and other biodegradable polymers, including starch, cellulose derivatives, lignin, poly (lactic acid), polycaprolactone and mixtures of different types are summarized PHA. Polyhydroxybutyrate, the PHA most studied and characterized, has mechanical properties similar to polypropylene and polystyrene, having a better performance as a barrier to passage of oxygen and UV light; It is waterproof, heat and water vapor permeability is three times lower than polypropylene.
Depending on the microorganism and growth conditions, the PHA can consist of units of 3 or 4 carbons up to 14 or 16 carbons. Given the stereospecificity of hydroxyalkanoate monomeric units microbial enzymes all exhibit D- configuration (-). Functionalizing PHAs by chemical modification described with respect to two major approaches to synthesis: block copolymerization and graft copolymerization. The PHA has disadvantages inherent high crystallinity (> 70%), including its fragility and the very limited working range because its melting temperature (approx. 179 ° C) is close to degradation (200 ° C) . To expand the range of operating temperatures, improve processing, impact strength, flexibility and reduce the barrier propertiesIt is better to use poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) copolymer. This is important since avoiding the use of toxic and expensive precursors to produce the copolymer. The expanded use of PHAs modified as engineering materials and biomedical importance in different areas is also addressed.
|
|

|
| Chemicals properties PHA |
|
|
Various PHAs that have been identified are mainly linear polyesters from start to finish monomers compounds 3-hydroxy fatty acids. In these polymers the carboxyl group of a monomer forms an ester with the hydroxyl group of the neighboring monomer link. They are waterproof and ultraviolet radiation and have very low permeability to oxygen and moisture, are particularly suitable as food packaging.
|
|

|
| PHA Processability |
|
|
PHAs are processed according to their properties, but mainly according to the chemical composition and molecular weight can be processed into a variety of finished including films, sheets, printed products, fibers, elastic, painted articles, nonwoven products.
In general: Low comonomer content and low molecular weight , PHA, are suitable for injection molding. A weight average molecular weight , the material is suitable for fiber spinning. With the increase in the percentage of comonomer and molecular weight average (600,000), and applications include cast film resins. Blown films and require at least one comonomer percentage of 10% and a high molecular weight (700,000). Comonomer content with more than 15% of PHA are soft and elastic, finding application in adhesives and elastic films.
| | 
|
| PHA Polimerization |
|
|
Production in vitroPHA production in vitro is performed in a cell free system, from monomers such as: lactones, hydroxyalkanoic acids or synthetic thioester 3-hydroxyacyl-CoA and using enzymes such as lipases, esterases or some proteases for synthesis .
This method has the advantage of not producing by -products, such as other compounds metabolic, which then have to be removed. They can be obtained pure polymers and can polymerize specific monomers not synthesized naturally. On the other hand the disadvantages include low stability (high cost) polymerizing enzymes and the use of relatively expensive substrates. Currently polymers synthesized by this route are used only for research.
Production in vivo PHA production in vivo can be given in two ways: by fermentation of substrates by microorganisms and through genetically modified plants.
Commonly produced PHA is the fermentative production of poly-beta-hydroxybutyrate (poly-3-hydroxybutyrate, P3HB) having from 1000-30000 hydroxy fatty acid monomers. In the industrial production of PHAs, the polyester is extracted and purified from bacteria by optimizing the fermentation conditions or microbial sugar glucose
Production in genetically modified plantsHave developed technologies PHA production by plant tissues GM farms, PHA accumulation occurs in both seeds and leaves, which through photosynthesis using carbon dioxide and water as raw material for PHA production |
| 
|
| PHA Applications |
|
|
The polyhydroxyalkanoates are versatile biopolymer with various applications in industries such as: pharmaceutical, biomedical, food, packaging, among others. Although not yet marketed or have very high global demand, some industrial applications for PHA have already been described, including the manufacture of thin coating films; binders in ink formulations based on water; as a source of chiral monomers for the synthesis of active compounds and as a support for tissue engineering and temporary medical implants. PHAs can be used in flexible films of varying thicknesses, including membranes semipermeable, filaments, fibers, packaging materials, gels and adhesives.Furthermore, they can be used on the cover of fibrous materials such as paper or paperboard from the aqueous latex form. Thus, given its high water resistance, is covered paper or paperboard protects against deterioration caused by moisture
| |
|
|
|
|
|
|