Reticulation
Additives
| Vulcanization | ||
Vulcanization is a chemical process to convert natural rubber or related polymers into more durable materials by heating them with sulfur (Peroxide, Metal Oxides, Acetoxysilane, Epoxide) or other equivalent healers or accelerators. These additives modify the polymer by forming crosslinks (bridges) between individual polymer chains. Vulcanized materials are less sticky and have superior mechanical properties. The term "vulcanized fiber" refers to cellulose that has been treated in a zinc chloride solution to crosslink the cellulose fibers. Unvulcanized rubber only has a highly elastic gummy phase, so it is in an amorphous state and has no capacity for use. After the vulcanizing agent, the accelerator and other additives are mixed with rubber molecules for vulcanization over a certain time, temperature and pressure, a cross-linked network structure is formed. This three-dimensional structure puts an end to the amorphous state of rubber, and gives rubber superior resistance to abrasion, modulus of elasticity and other excellent properties in general. This structure is a chemical cross-linking of molecules that can not be destroyed by temperature, which results in the difficulty of rubber in recycling and reprocessing. However, the physical three-dimensional structure of TPE is formed by the rigid phase in the vitreous state and the gummy phase in the highly elastic state at room temperature, and can be destroyed by increasing the temperature. As a result, TPE can be used and processed repeatedly, and it is easy to recycle. | ||
| Vulcanization causes | ||
Vulcanization causes profound changes at the molecular level. The long rubber molecules become cross-linked together with the junctions (cross-links) spaced along the polymer chains, with the average distance between the junctions and the 4,000 to 10,000 daltons. As a result of this network formation, it can not be processed by any means that requires it to flow, for example, in a mixer, in an extruder, in a mill, in a fuel transfer, or during modeling, shaping or molding. It is essential that the vulcanization occurs only after the rubber article is in its final form. |  | |
| Hardening of a thermostable resin | ||
Hardening of a thermostable resin by chemical reaction of condensation or addition that produces the cross-linking or cross-linking of its chains, giving rise to a product that does not change its properties. It may be accompanied by the addition of crosslinking agents, with or without the addition of catalysts, with or without application of heat and / or pressure. | ||
| Modulo | ||
The static module increases to a greater extent than the dynamic module. The dynamic modulus is usually measured with the imposition of a small sinusoidal voltage at a frequency of 1-100 Hz. The modulus is a composite of viscous and elastic behavior, while the static modulus is largely a measure of the elastic component of behavior Rheological If the polymer is cross-linked, and the temperature is sufficiently high, the elastic modulus grows linearly as the temperature increases, until decomposition, as is the behavior of the vulcanized rubbers. |  | |
| Hysteresis | ||
Hysteresis is the ratio of the viscous or speed-dependent component to the elastic component of the resistance to deformation. It is also a measure of the deformation energy that is not stored (or supported by the elastic network) but converted to heat. The vulcanization causes a compensation of elasticity due to viscous or plastic behavior. Resistance to tearing, fatigue and resistance. The values of these properties increase with small amounts of crosslinking, but are reduced to a greater extent. | ||
| Sulfur vulcanization without accelerator | ||
Initially, the vulcanization was performed using elemental sulfur at a concentration of 8 parts per 100 parts of rubber (phr), it took 5 hours to 140 ~. The addition of zinc oxide reduced the time to 3 hours and the use of accelerators in such a low concentrate reduces the time from 1 to 3 minutes. As a result, the vulcanization of elastomers by sulfur without accelerator no longer has much commercial importance. | 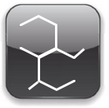 | |
| Sulfur vulcanization with accelerator | ||
By adding sulfur and N- (cyclohexylthio) phthalimide (CTP) whose small concentrations were used together with benzothiazolesulfenamide accelerators, a faster vulcanization is obtained. Vulcanization with accelerated sulfur is the most used method. For many applications, it is the only rapid crosslinking technique that, in fact, can provide the delayed action required for processing, shaping and forming prior to the formation of the intractable vulcanized network. It is used to vulcanize natural rubber (NR), synthetic isoprene rubber (IR), styrene-butadiene rubber (SBR), nitrile rubber (NBR), butyl rubber (IIR), chlorobutyl rubber (CIIR), bromobutyl rubber ( BIIR) and ethylene-propylene-diene monomer rubber (EPDM). | ||
| Types of curing systems are commonly used, they are: | ||
| ||
| Dynamic vulcanization | ||
Dynamic vulcanization is a process by which a crosslinkable material is cured in situ during a melt mixing process. The result is a dispersion of micrometer scale particles of crosslinked rubber dispersed in a polymeric matrix. With a significant tangle of the matrix polymer. on the surface of the cured particles. |  | |
