PA
Thermoplastics > Polyamides
| Polyamides | ||||||||
| History of polyamide | ||||||||
Two professors of chemistry, one from New York and one from London, at the same time, were investigating the behavior of simple molecules that together can form giant molecules such as molecules that contained amino groups and carboxyl groups, the result was a molecule with large rings and it was called Nylon or polyamide. When adipic acid and hexamethylenediamine were mixed, they were condensed to give a polymer with a silk-like structure. When these two reagents are mixed, a proton transfer reaction occurs which results in a white solid called Nylon salt. When it is heated to 250 ° C, water is removed in gaseous form and molten Nylon is obtained. Molten Nylon is molded to its solid form or extruded through a spinner to obtain a fiber. This first completely synthetic fiber was called nylon (polyamdia 6.6). The polyamides began to be used as synthetic fibers, although they have ended up being used in the manufacture of any plastic material. Since hexamethylenediamine and adipic acid each have six carbon atoms in their molecule, the new substance was called "polyamide 6.6". It is believed that his name is a play on words, referring to NY (New York) and Lon (London), two cities that conjugate in English result in NyLon, since it would be discovered by two researchers who lived one in each city. Another legend attributes the name to abbreviations of exclamations like "Now You Lousy Old Nipponese" (or "Now You Look Old Nippon" or "Now You Loose Old Nippon") against the Japanese when being a substitute of the silk that is had imported from China occupied by the Japanese in World War II. | ||||||||
| The differences between PA 6 and PA 6.6 | ||||||||
PA 6 and PA 6.6 are very similar, both are partially crystalline and have the same base, but they have a different order in their individual molecules. The different dispositions of the molecules cause us to find more mechanical benefits in PA 6.6. Its basic properties such as chemical resistance are the same, have good sliding and cushioning properties, good abrasion and good impact resistance, both are also resistant to weak alkalis, fats and oils. PA 6.6 absorbs less moisture and therefore is more stable while the advantages of PA 6, are seen when reinforced with fiberglass, in fact has a better surface finish. In addition, the lower processing temperatures of PA 6 (222 ° C versus 265 ° C of PA 6.6) reduce the energy consumption leading to lower production costs compared to PA 6.6. Due to the higher moisture absorption, PA 6 has a higher impact resistance than PA 6.6. By using glass fibers, the mechanical properties can be improved and the operating temperatures can be increased. Mexpolimeros, offers polyamides with fiberglass reinforcements and mineral fillers up to 60%. We produce high impact polyamides, stabilized against heat and hydrolysis, of all the desired colors. | ||||||||
| Classification of polyamides | ||||||||
Polyamides are chemically characterized by their macromolecular structure, composed of an amide group (-NH-CO-), which is formed by the reaction of a carboxylic group with an amino group as a recurring functional unit that provides the chemical properties specific to the products. endings They are especially versatile due to their ease of synthesis and enjoy exceptional mechanical and thermal properties. The abbreviation PA followed by a number PAn, indicates the number of carbon atoms of those of acid, if the numbers are two, the first represents the number of carbon atoms of the amine, while the second number represents the carbon atoms of the acid bicarbossilico polyamides. They are high molecular weight compounds with lineare structure. The presence of amide groups capable of forming groups with hydrogen defines the physical and chemical properties that are common to all polyamides. They provide excellent mechanical, physical and thermal properties, the thermal properties of polyamides differ between them for ductility and rigidity, strength and strength characteristics, and allow processing with almost all systems in use for thermoplastic materials. From a structural point of view, they are divided into two main types: Derived from amino acids or lactams (-NH-R-CO-) n AB Derivatives of diamines and dicarboxylic acids (-NH-R-NH-CO-R'-CO-) n AABB | ||||||||
| Polyamides derived from lactams | ||||||||
The polyamides derived from the polymerization of lactams, through the ring opening, or the polycondensation of amino acids, in which A is defined as the first functional group (amine) and for the second functional B (carboxylic acid). Polyamide 4 or polypyrrolidone (amminobutanoic acid lactam 4) Polycaprolactam or polyamide 6 Polyamide 11 or poly-wamminoundecanoic acid Polyamide 12 or polylaurolattame or poly-wamminododecanoic Polyamide 69 from azelaic acid Per AABB acid, means that the polyamides obtained by polycondensation of a diamine (AA) with a dicarboxylic acid (BB). | 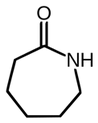 | |||||||
| Polyamides derived from diamines and dicarboxylic acids | ||||||||
In the case of simple linear monomers the first number refers to the number of carbon atoms in the diamine and the second number refers to the number of carbon atoms in the dicarboxylic acid; in the case of simple linear monomers the first number refers to the number of carbon atoms in the diamine and the second number refers to the number of carbon atoms in the dicarboxylic acid;
for non-linear cases, with more complex molecules or at least non-linear simple are used, instead of numbers, literal abbreviations that indicate the monomer or the structural comonomer: MXD for m-xylylenediamine, | ||||||||
| Aromatic polyamides | ||||||||
Aromatic polyamides, often called aramides, have a higher resistance to heat in flame solvents and greater dimensional stability than all aliphatic polyamides, but they are much more expensive, more difficult to produce and work. Polyamides have several advantages over other engineering plastics. For example, they are more resistant to alkaline hydrolysis than polyesters but do not resist acid hydrolysis. They also have a better resistance to organic liquid solvent than PET and PC. | ||||||||
| Thermal properties | ||||||||
The properties of the Polyamides are related to the presence of the amide group in the chain that allows the formation of strong interchain hydrogen bonds. The melting temperatures of PAs vary according to their molecular structure, within a very high temperature range (from 178 ° C for PA12 to approximately 295 ° C for partially aromatic PA, up to values above 500 ° C for fully aromatic. | ||||||||
| Aliphatic polyamides | ||||||||
PA 46 PA 6 PA 6.6 PA 11 PA 12 PA69 PA 410 PA 610 PA 612 |  | |||||||
| Amorphous aliphatic polyamides | ||||||||
PA MACM 12 | ||||||||
| Amorphous semi-aliphatic polyamides | ||||||||
PA6 L, PA4 T, PA4 L, PA MXD6 are partially aliphatic polyamides, thanks to the presence of an aromatic monomer, they can work at higher temperatures, high rigidity, good dimensional stability and low shrinkage. | ||||||||
| Semi-aromatic polyamides PPA | ||||||||
PA, PA9T, PA MXD6 or m-xylylenadipamide, PA6 T and HTN have a high melting point, low moisture absorption, therefore improved stability of the month, short cycles, good chemical resistance, excellent surface appearance, excellent mechanical properties. | ||||||||
| Aromatic polyamides PPA | ||||||||
Also called polyphthalamide (PPA), of polyphthalamide polyamide, of commercial interest we have 2 types: The first is derived from the polycondensation of isophthalic acid and m-phenylenediamine. It has a large flame retardant capacity and is used for the construction of fire fighting suits. The second is derived from the polycondensation of terephthalic acid and p-phenylenediamine. Its main characteristic is the great resistance to tractions and impacts. It is used in a variety of fields, from ropes for mountaineers to bulletproof vests. Aromatic polyamides have a higher resistance to oxidation, but have a lower resistance to flammability. Polyphthalamide PPA is a high performance synthetic thermoplastic resin from the polyamide family that is used to replace metals in automotive high temperature applications. | ||||||||
| Amorphous branched aromatic polyamides | ||||||||
PA6.3 T is transparent with excellent physical-mechanical properties, the amorphous structure also reduces shrinkage and, consequently, warping | ||||||||
| Crystallization rate | ||||||||
The crystallization rate of the polyamides is very important at the application level and, although it is generally quite high, it is often necessary to increase it further by the use of nucleating agents. The crystallization rate is also inversely proportional to the molecular weight following the general rule of all semicrystalline polymers. The rapid cooling of molten polymer, such as those that occur in most transformations (molding and extrusion are typical examples), block the spherulite growth process to a level that depends on the type of polyamide and obviously on the cooling rate . This fact creates problems in cases where the product obtained by rapid cooling does not relax enough and in the case of polyamides 6 and 66 the reabsorption of moisture, the reduction of the glass transition point, creates post-crystallization problems of No despicable way and that must be taken into account. Above all, the absorption of water generates remarkable changes also of the mechanical characteristics and an expansion of the piece that goes in the opposite direction to the secondary crystallization, also among other things favored by the absorption of water, which makes the problem even more complex. | ||||||||
| Humidity polyamide | ||||||||
The negative aspect, in terms of mechanical strength, is due to the fact that polyamides with a higher concentration of chain amino groups tend to absorb more water. This implies a decrease in the mechanical characteristics at the level of traction because the water, a highly effective dipole, which is interposed between the hydrogen bonds causes the chains to slide more and therefore reduces the glass transition temperature of the material . Polyamides with a lower CH2 / CONH ratio are those that absorb more water and, therefore, will vary more their mechanical characteristics by conditioning. It is very important to take into account your moisture level when converting polyamides. This information is fundamental from the point of view of the manufacturer of the material and the user, so fundamental that it is considered an acquired element and also the way of packaging these materials is such that guarantees the maintenance of the original moisture content for many months. This extreme sensitivity to water content is due to the fact that a water content greater than the solubility of the latter in the molten polymer would create several problems during the process. | ||||||||
| Molecular weight of polyamides | ||||||||
Polyamides in general do not vary their molecular weight over a very wide range, as can happen for polyaddition or coordinated anionic polymerization polymers, in particular for polyolefins and derivatives. The range of molecular weights, foreseen as numerical averages - average of Mn - varies from a minimum of 15,000 to a maximum of 60000. The lowest molecular weights are not extrudable in the molten state continuously, while for molecular weights above 60,000 they are limitations due to the need to work with water contents so low that they are not easily reachable or, if they were, at values that are difficult to maintain (H20 <100 ppm). Regarding the mechanical characteristics, we can say that the increase in molecular weight leads to an increase in the impact resistance of the material and makes it suitable for certain applications for which resilience is an important factor. | ||||||||
| Reinforced polyamides | ||||||||
The bases used to obtain reinforced polyamides, contrary to what has been said previously, it is preferable that they are unfinished. In fact, it has been shown that the mechanical characteristics and especially the impact resistance are influenced by the termination. The explanation of this behavior can be found perhaps in the fact that the terminal amino groups could react with the basic groups of the fiberglass increasing the curling of the polymer to the fiber itself. In addition, the processes of mechanical-chemical degradation and heat that can take place in double-screw extruders, which also move at a fairly high number of revolutions, can be compensated for by the increase in unfinished polymer viscosity, at least in the area before the entrance of fiber glass; in fact, once the terminal amino groups have reacted with the fiber, the condensation subsequent to the polymer is blocked or slowed down considerably. | ||||||||
