Surface tension
Polypedia > Properties of polymers > Physical properties
| Surface tension | ||
The cohesive forces between the molecules of a liquid are responsible for the phenomenon known as surface tension. All molecules that are composed of a liquid substance interact in some way with each other and attract each other. Of the molecules of a liquid, only those that are on the surface, are subject to intermolecular forces that are not symmetrical, as happens with the molecules that are inside the liquid. The result is that the surface of a liquid is in a particular state, and all the liquid behaves as if it were wrapped by a very thin and stretched elastic film. By surface tension, property of a liquid that makes it behave as if its surface were encased in an elastic sheet The attractive forces between the molecules of a liquid can be considered as residual electrostatic forces and are sometimes called van der Waals forces or van der Waals adhesion. | 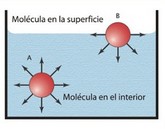 | |
Surface tension or surface energy | ||
It is a thermodynamic quantity defined as work necessary to isothermally increase the surface area of a liquid. The surface tension is a direct measure of the intermolecular forces at the level of the surface. Due to surface tension the tendency of liquids is to reduce their energy as much as possible, then to form drops. The surface tension varies with the temperature, the composition of the flow and the interactions between the alloy of the substrates. For these reasons, the surface tension of brazing alloys is not known precisely. In general, the surface tension tends to be lower in the air than in an inert atmosphere. The action of the environment is generally determined by the absorption of vapor on the polymeric surface, and it is what reduces the surface tension. If the surface tension of a liquid, placed on a surface, is greater than that of the solid, it forms a contact angle between the solid phase and the liquid phase. When the same liquid is placed in super? Ci With the increase in surface tension, the contact angle decreases. |  | |
| La tensión superficial, se mide normalmente en dinas/cm | ||
The force that is required (in dynes) to break a 1 cm film. of length. The surface energy in ergs per square centimeter can be established in an equivalent way. The surface tension depends on the nature of each liquid. Surface tension is defined in physics as the amount of energy needed to increase the surface of a liquid and counteract that force of attraction inward. Water at 20 ° C has a surface tension of 72.8 dynes / cm compared with 22.3 for ethyl alcohol and 465 for mercury. |  | |
| Bagnabilità | ||
A key feature to obtain a good bond between two polymers is the wettability, that is, the capacity of the molten polymer in the liquid phase to flow and diffuse during the overinjection process. A measure of wettability is the contact angle formed at the junction of a solid and a liquid in a particular environment. In thermodynamic terms, good wettability occurs if there is a net decrease in total free energy when the new interface between the two polymers is formed. | ||
| Tintas de prueba | ||
In order to make possible the printing, painting and gluing of particularly "difficult" plastic materials (such as polyolefins and / or certain technopolymers), adequate pretreatment is necessary (for example, with atmospheric pressure or vacuum plasma) so that the surface tension reaches a certain value. The measurement of the surface tension is done by measuring the contact angle of a fluid on the surface of the product or, more quickly and easily, with the use of wetting measurement inks. The measure is necessary: - before painting: water-based paints require a wettability of at least 45 mN / m (dyne) - Before printing: the adhesion of the printing inks requires a wettability of approx. 38 mN / m - Before gluing: at least 42 mN / m - After the treatment to verify that the above parameters have been reached. It must be remembered that a good wettability of the surfaces to be glued, printed and painted is a necessary condition, but not always sufficient, and other factors that may condition adhesion sometimes intervene. We indicate the intrinsic surface tensions of some untreated plastics: Polypropylene 29 mN / m (dyne) Polyethylene 31 Polystyrene 35 Polyamide <37 PBT 34 Silicone 23 PVC 38 PMMA <37 ABS 36 Mexpolimeros supplies inks for the wettability test in packages of 8 bottles of 30 ml, in the standard version from 30,34,38,40,44,46,52,58 mN / m. On request also different gradations. | 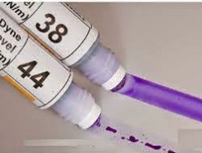 | |
| Did you know that hot water freezes faster than cold water? | ||
The tension of the surface depends on the nature of each liquid, the environment that surrounds it and the temperature. When heat is applied to a liquid and the temperature increases, the surface tension drops while the cohesion forces are also reduced. |
